NanoMicrobiol NanoBiotechnol, 2023 (2), 202323
DOI:
Original Article
Bactericidal effects of biosynthesized silver nanoparticles against Pseudomonas aeruginosa
Alireza Ebrahiminezhad 1*
1 Biotechnology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
* Correspondence: a_ebrahimi@sums.ac.ir
Abstract
Silver nanoparticles is one of the most potent and most studied antimicrobial nanoparticles. These particles are now employed as antimicrobial agent in the commercial products. Siler nanoparticles are also considered safe to be employed in the topical pharmaceutical products. Due to these widespread applications, diverse techniques were developed for the fabrication of silver nanoparticles via physical, chemical and biological approaches. Induction of oxidation stress due to the production of reactive oxygen species (ROS) and release of silver ions is considered as the main mechanism for antimicrobial action of silver nanoparticles. Antimicrobial effect can be due to bacteriostatic or bacteriostatic effects of nanoparticles. In the current experiment this issue was evaluated for the biosynthesized silver nanoparticles. Silver nanoparticles were synthesized using secretory compounds from microalgae Chlorella vulgaris. Antimicrobial potency against Pseudomonas aeruginosa was tested via agar diffusion assay. After 18 h incubation a growth inhibition zone with 20 mm in diameter was appeared. Bacterial cells viability assay indicated that all P. aeruginosa cell were dead after 18 h exposure to silver nanoparticles. These findings can pave the way toward increasing our knowledge about antimicrobial action of silver nanoparticles.
Keywords: AgNPs; Bacteriostatic; Bactericidal; Microalgae; Secretory compound
1 Introduction
Silver nanoparticles are clusters of silver atoms that range in diameter from 1 to 100 nanometers. Silver nanoparticles can be fabricated by a variety of physical, chemical, and biological techniques. Synthesis technique can affect characteristic features of the resulted particles such as average diameter, particle size distribution, shape of nanoparticles, stability, crystallinity, and purity. In chemical technique some coating agents such as citrate can be employed to prevent aggregation and sedimentation of nanoparticles. In biosynthesis methods, silver nitrate solution is usually added to the microbial culture superintendent and secretory reducing agents such as hydroquinones in microbial supernatant reduce silver ions into silver nanoparticles. Silver nanoparticles are nano-sized structures that are formed by bonding silver atoms to each other (metal bonding). At the nanoscale, particles exhibit different physical, chemical, and optical properties due to the dominance of quantum mechanics (1). Silver nanoparticles have a wide range of applications, including electronics, biosensors, clothing, dye industries, food industries, sunscreens, cosmetics, medical devices, and antimicrobial agents (2).
Mechanism for antimicrobial action in silver nanoparticles is under study but some previous reports indicated that induction of oxidative stress is the main antimicrobial mechanism. Antimicrobial stress can be inducted via production of reactive oxygen species or release of silver ions (3). But the main question is about the fate of microbial cells after exposure to the silver nanoparticles. If silver nanoparticles can kill bacterial cells or just halt their growth. In the current experiment silver nanoparticles were synthesized in a biological manner using secretory compound from a micro alga, Chlorella vulgaris (4). The particles were exposed to Pseudomonas aeruginosa and after 18 h exposure, viability of bacterial cells were evaluated. These finding can light our way for more insight about the mechanism of antimicrobial action of silver nanoparticles.
2 Experimental
2.1 Biosynthesis of silver nanoparticles
50 ml of the alga culture containing 107 cells per ml was prepared and kept in the culture room under light. After 20 days, medium was centrifuged (10000 rpm for 10 min) and supernatant was used for the synthesis of silver nanoparticles. The supernatant solution with a ratio of 1:2 was added to silver nitrate solution with a final concentration of 5 mM and was stored at 25 °C for 24 hours. Synthesized nanoparticles were harvested (10000 rpm for 20 min) and sediment was washed three times with deionized water.
2.2 Characterization of silver nanoparticles
Resulted suspension was diluted 10 times and optical spectra was recorded in the range of 300 to 700 nm using a microplate reader (Epoch2, BioTek, USA). A drop of silver nanoparticles was drip on a glass coverslip and dried at ambient atmosphere for X-ray diffractometry analysis (XRD, Siemens D500, Germany). Also particles were visualized via transmission electron microscopy (Philips CM 10, TEM, operated at HT 100 Kv.).
2.3 Antimicrobial evaluations
Antimicrobial potency of the synthesized particles was examined against P. aeruginosa (ATCC 27853) via agar well diffusion assay. The test was performed based on the performance standards for antimicrobial disk susceptibility test; approved standard-eleventh edition (CLSI, M02-A11) with some modification to well diffusion test (5). In this regard bacteria were cultured on the Mueller-Hinton agar plate for 24 hours. A colony was transferred to normal saline to reach turbidity equal to a 0.5 McFarland standard. This suspension was used as inoculum and transferred on the agar plate. AgNPs were loaded to a well with six mm in diameter. After 18 h incubation at 37°C, the diameter of growth inhibition zone was measured.
2.4 Viability assay
To evaluated viability of the bacterial cells, a piece of agar medium was cut from the inhibition zone and transferred to fresh media. After 24 h incubation at 37°C, possible growth of bacterial cells was evaluated via optical turbidity.
3 Results and discussion
3.1 Biosynthesis and characterization of silver nanoparticles
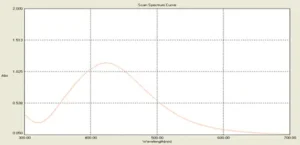
After adding silver nitrate to the surface solution of the microalgae medium the color of the solution changed to red-brown. The intensity of color gradually increased and after 24 hours turned to a completely dark brown. After the incubation period, the resulting suspension optical absorption was measured in the region of 300 to 700 nm and the peak of silver nanoparticles was observed in 428 nm (Fig. 1).
Fig. 1. UV-vis spectra of the biosynthesized silver nanoparticles with an absorption peak at 428 nm
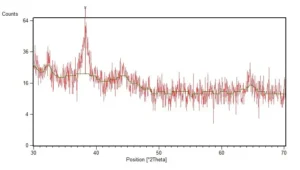
XRD spectra of the synthesized AgNPs were depicted as Fig. 2. Diffraction pattern demonstrate a diffraction peak at 38.25 of 2-theta degree which is characteristic peak of silver crystals. TEM micrograph of the synthesized nanoparticles was shown in the Fig. 3. Most of the synthesized nanoparticles were spherical in shape. According to the analysis which carried out on the TEM micrographs, the size of nanoparticles was between 5 and 14 nm and the average diameter of nanoparticles was 7 nm.
Fig. 2. XRD spectra of the biosynthesized AgNPs, characteristic diffraction peak of silver crystals was recorded at 38.25 of 2-theta degree
Fig. 3. TEM micrograph of the biosynthesized silver nanoparticles
3.2 Antimicrobial Evaluations
Antimicrobial evaluations were done via agar diffusion assay. After 18 h incubation period, growth inhibition zone was measured to be 20 mm, indicating the potency of examined silver nanoparticles against P. aeruginosa (Fig. 4). Transferee of P. aeruginosa cells which were exposed to silver nanoparticles to fresh media was resulted in any development of turbidity. This observation can approve that biosynthesized silver nanoparticles were able to kill bacterial cells of P. aeruginosa in 18 h exposure time.
Fig. 4. Growth inhibition zone of silver nanoparticles against P. aeruginosa
Conclusion
Due to vide applications of silver nanoparticles in different fields, various techniques for the fabrication of silver nanoparticles were developed over last decade. Nowadays, antimicrobial effect of silver nanoparticles is a confirmed fact and silver nanoparticles are employed in commercial products as a safe antimicrobial compound. It is to some extent approved that silver nanoparticles develop oxidative stress on the microbial cells and hence antimicrobial properties. In the present work an experiment was developed to determine the fate of bacterial cells after exposure to silver nanoparticles. It was found that silver nanoparticles are so potent antimicrobial agent that can kill all bacterial cells which were exposed to. These finding can lead us for further studies and applications of silver nanoparticles as potent antimicrobial compound.
References
-
- Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580-8.
- Ahamed M, AlSalhi MS, Siddiqui M. Silver nanoparticle applications and human health. Clin Chim Acta. 2010;411(23):1841-8.
- Xiu Z-m, Zhang Q-b, Puppala HL, Colvin VL, Alvarez PJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12(8):4271-5.
- Ebrahiminezhad A, Bagheri M, Taghizadeh S, Berenjian A, Ghasemi Y. Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv Nat Sci. 2016;7:015018.
- (CLSI) CaLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Eleventh Edition. PA 19087 USA: CLSI; 2012. p. 58.