NanoMicrobiol NanoBiotechnol, 2022 (2), 202226
DOI:
Short Review
Gold nanoparticles and visual detection of Leishmania
Somayeh Daris 1
1 Department of Medical Biotechnology, Scholl of Medicine, Fasa University of Medical Sciences, Fasa, Iran
* Correspondence: s.daris@fums.ac.ir
Abstract
Leishmaniosis is a large group of diseases that cause by Leishmania spp. Definitive diagnosis is based on clinical and laboratory tests. Common methods of ensuring the diagnosis of visceral Leishmania are serological and parasitology tests which are not so successful exams. In recent years, diagnosis based on polymerase chain reaction (PCR) has overcome the old methods and increased sensitivity and less invasive sampling. But PCR comes with some limitation problems such as cost, need for specific equipment, and trained users. Gold nanoparticles can make the detection of Leishmania, fast, easy, and economic. Using gold nanoparticles, Leishmania can be detected in any sample simply by a change in the color of reaction that can be identified with naked eye. This novel technology can be promising in the case of complicated diagnostic tests and make them available in rural and remote areas.
Keywords: Colorimetric; DNA probe; Gold nanoparticles; Leishmania diagnostic; Nanosensors
1 Introduction
Leishmaniosis is a large group of diseases that cause by Leishmania spp. That is a protozoan in the trypanozomatidae family. The difference between skin, mucosal, dermal or visceral disease depends on the host immune response and specific strains of Leishmania. Leishmaniosis is endemic in 98 countries and more than 350 million people are exposed to the infection. About half million new cases are added each year, with 50,000 fatal and 1.5 million cases of skin leishmaniasis occurring annually (1-4). Leishmania parasite is transmitted to humans by biting phelebotomine sand fly. Visceral leishmaniosis is caused by Leishmania infantum or Leishmania shagassy in Iran, which causes kala-azar disease. It is often fatal and severe (3). Visceral leishmaniosis is an endemic zoonotic disease in northwest and south of Iran (5-8). Visceral leishmaniosis is the second leading cause of death and the 4th cause of morbidity among tropical diseases with 20,000 to 40,000 deaths per year and over 2 million dalys (disability_ adjusted life years). Since visceral leishmaniasis is fatal in the absence of treatment, it is important to diagnose the disease quickly and accurately. Similarly of the symptoms with other diseases makes the diagnosis of leishmaniosis harder and more complicated (9-11).
2 Common diagnosis techniques
Definitive diagnosis is based on clinical and laboratory tests. Common methods of ensuring the diagnosis of visceral leishmaniosis are serological and parasitology tests. Parasitological studies directly show the presence of Leishmania infantum in liver, spleen, lymph nodes, and bone marrow aspirations. Although many patients are not confident in diagnosis due to the low sensitivity of microscopic studies, serological tests such as indirect immunofluorescence assay (IFA) and enzyme-linked immunosorbent assay (ELISA) have false negative results and also you can expect crossovers with other diseases. The sensitivity of diagnosis in the use of aspiration smears varies from different tissues (e.g. spleen, liver, and bone marrow). Results of tissues examinations have shown that the rate of success in diagnosis with spleen aspiration is 98.95%, with lymph node aspiration is 69.52%, and with liver and bone marrow aspiration is 91.76% and 89.52%, respectively (9, 12-18).
In general, serological methods are of little value in medical diagnostics because they remain positive for months or years after clinical treatment (19, 20). Direct examination is inexpensive and easy, but when the number of parasites in the tissue is low, it is insensitive and requires a very high skill. Parasite-based tests are laborious and require special conditions and also require longer time to obtain results (21). Indirect immune fluorescence assay test is a routine diagnostic method for canine visceral leishmaniosis. In this case, asymptomatic dogs may have false negative results, and a cross reaction may occur with other Leishmania species or even other diseases (22, 23). Studies have shown that in endemic areas, infected animals, especially those that are asymptomatic, often remain seronegative or borderline. Serological tests cannot be used in cases of recurrence because antibodies remain for a long time after treatment. Also many people in endemic areas have antibodies against the disease due to high contact duration and asymptomatic infections (24-26). In general, this suggests that serology may not be a good choice for determining Leishmania contaminations.
Culture in a specific medium is another method of parasitological diagnosis that is not used routinely because it requires about 30-day growth period (27). However, gold standard for visceral leishmaniasis diagnosis is identifying parasites in the culture of spleen aspirates that requires surgery and causes bleeding. So, bone marrow aspiration and lymph nodes are used for parasitological diagnosis in most cases (28).
3 Biotechnological diagnosis
In recent years, PCR based diagnosis was introduced as a sensitive technique and has overcome the old methods in Leishmania diagnosis. Beside increased sensitivity and specificity, sample preparation for PCR based diagnosis is more preferred. PCR tests requires less invasive sampling because peripheral blood samples can be used in this method (29, 30). The sensitivity of PCR methods varies depending on the selected target sequence. Sensitivity of PCR using blood had a range between 96.70%. Real_Time PCR method increases the sensitivity of diagnosis, especially for blood samples. Real_time PCR has advantages over conventional PCR, one of these advantages is that the test time is shorter because electrophoresis is not required. Real_time PCR is sensitive enough to detect 0.001 parasites in each human blood sample reaction (31-33). But problems such as cost and need for specific equipment such as thermal cycler, skilled operator, and a system for diagnosis and analysis forced us to look for an easy and low cost solution.
4 Nanotechnological diagnosis
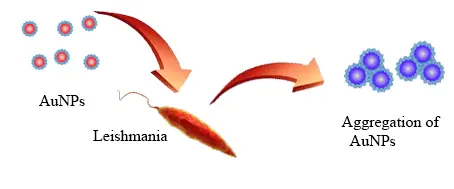
With advances in nanotechnology and the use of nanoparticles, especially gold nanoparticles, in biosensors, detection of Leishmania parasite in medical samples is possible in a very low cost, fast and easy manner. Gold nanoparticles based nanosensors are using DNA fragments as biomarkers and developed to detect the target DNA calorimetrically to be easily detectable by naked eye. Detection of pathogens by using nanoparticles is based on the accumulation of engineered gold nanoparticles during the absence of target DNA fragment and changing the color of the solution (34). Two different methods were developed based on calorimetry methods to diagnose microbial DNA, amplification and non-amplification detection technologies. In the non-amplification method, probe modified gold nanoparticles are used, which complements a piece of DNA. In the absence of complementary DNA, the probe modified gold nanoparticles precipitates in the presence of acid, which causes the color to change from red to purple that can be identified with the eye. In the presence of target DNA, no sedimentation is occur and the color of the solution remains red (35). For instance, Margarita Andreadou et al. designed four oligonucleotides in 2014 that were complemented fragments of the KDNA in Lishmania spp. This piece is very protected among Leishmania species. In this study, in the absence of Lishmania DNA, the probe modified gold nanoparticles accumulated and the mixture color is changed from red to purple. While in the presence of target DNA, no change in the color can be observed. Minimal detectable DNA sample was reported to be 11.5 ng/µL and the reliability of the test was 100%. The sensitivity and specificity of this method was reported as 92% and 100%, respectively (36).
In the DNA amplification method, the target DNA fragment is amplified by PCR. In this way, primers connected to thiol are used for amplification. Gold nanoparticles are added to the PCR solution, followed by acid, which induces the accumulation of gold nanoparticles. In the presence of the target DNA, the target piece is amplified, causing the distance of gold nanoparticles and the accumulation of nanoparticles does not take place, so there is no color change in the solution. In the absence of target DNA, primers modified nanoparticles easily connected to each other and causes visible change in the color of the solution. For instance, in an experiment that was conducted by Ye Lim Jung and her colleagues in 2010, they used thiol functionalized primers to detect Chlamydia infections. In this order acid and gold nanoparticles were added to PCR products, if the desired piece of DNA has been amplified, the color of the solution remains red, but in the absence of target DNA fragment the color of mixture changes to blue (37).
Application of gold nanoparticles was not restricted for Leishmania diagnosis. These particles were also employed for diagnosis of other infections. For example, in a study conducted by Bruno veigas et al. in 2015, a nanoprobe was used to detect two pathogens, Mycobacterium tuberculosis and Plasmodium sp. In this study, rpoB gene region was used for diagnosis of Mycobacterium and 18s rRNA gene region was used for Plasmodium diagnosis. This diagnostic technology is designed in such a way that the presence of each of these pathogens prevents the accumulation of probe modified gold nanoparticles and no change in the color is occur (38).
5 Conclusion
Traditional techniques for the detection of infections in medical samples are not perfect techniques and there is considerable drawback such as false reports and invasive sampling. Nowadays, new technologies come with valuable solutions. Developments in biotechnology provide PCR technique with improved accuracy and fast tests results. There is another gift by nanotechnology, nanosensors based on gold nanoparticles even make the job easier and faster. Now, gold nanoparticles based nanosensors can detect pathogens simply in a colorimetric reaction that can be identified with naked eye.
References
-
- Mokni M, editor Cutaneous leishmaniasis. Ann Dermatol Venereol; 2019.
- Clemente WT, Mourão PHO, Lopez-Medrano F, Schwartz BS, García-Donoso C, Torre-Cisneros J. Visceral and cutaneous leishmaniasis recommendations for solid organ transplant recipients and donors. Transplantation. 2018;102(2S):S8-S15.
- Minodier P, Parola P. Cutaneous leishmaniasis treatment. Travel medicine and infectious disease. 2007;5(3):150-8.
- Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22(12):552-7.
- Mohebali M. Visceral leishmaniasis in Iran: review of the epidemiological and clinical features. Iranian journal of parasitology. 2013;8(3):348.
- Norouzinezhad F, Ghaffari F, Norouzinejad A, Kaveh F, Gouya MM. Cutaneous leishmaniasis in Iran: results from an epidemiological study in urban and rural provinces. Asian Pacific journal of tropical biomedicine. 2016;6(7):614-9.
- Farahmand M, Nahrevanian H, Shirazi HA, Naeimi S, Farzanehnejad Z. An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran. The Brazilian Journal of Infectious Diseases. 2011;15(1):17-21.
- Holakouie-Naieni K, Mostafavi E, Boloorani AD, Mohebali M, Pakzad R. Spatial modeling of cutaneous leishmaniasis in Iran from 1983 to 2013. Acta Trop. 2017;166:67-73.
- Mondal S, Bhattacharya P, Ali N. Current diagnosis and treatment of visceral leishmaniasis. Expert review of anti-infective therapy. 2010;8(8):919-44.
- van Griensven J, Diro E. Visceral leishmaniasis: recent advances in diagnostics and treatment regimens. Infectious Disease Clinics. 2019;33(1):79-99.
- Ejazi SA, Ali N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert review of anti-infective therapy. 2013;11(1):79-98.
- Da Silva MRB, Stewart JM, Costa CHN. Sensitivity of bone marrow aspirates in the diagnosis of visceral leishmaniasis. The American journal of tropical medicine and hygiene. 2005;72(6):811-4.
- Weigle KA, de Davalos M, Heredia P, Molineros R, Saravia NG, D’alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. The American journal of tropical medicine and hygiene. 1987;36(3):489-96.
- Kumar A, Pandey SC, Samant M. A spotlight on the diagnostic methods of a fatal disease Visceral Leishmaniasis. Parasite Immunol. 2020;42(10):e12727.
- Romero GAS, Sampaio RNR, Macêdo VdO, Marsden PD. Sensitivity of a vacuum aspiratory culture technique for diagnosis of localized cutaneous leishmaniasis in an endemic area of Leishmania (Viannia) braziliensis transmission. Mem Inst Oswaldo Cruz. 1999;94:505-8.
- Luz ZMP, Silva ARd, Silva FdO, Caligiorne RB, Oliveira E, Rabello A. Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2009;104:62-6.
- Santos T, Carreira V, Ferrari H, Moreira M, Luvizotto MCR. Comparison of PCR with stained slides of bone marrow and lymph nodes aspirates with suspect diagnosis for leishmaniasis. Acta Trop. 2014;140:137-40.
- Ikeda-Garcia FA, Lopes RS, Marques FJ, de Lima VMF, Morinishi CK, Bonello FL, et al. Clinical and parasitological evaluation of dogs naturally infected by Leishmania (Leishmania) chagasi submitted to treatment with meglumine antimoniate. Vet Parasitol. 2007;143(3-4):254-9.
- Cota GF, De Sousa MR, Demarqui FN, Rabello A. The diagnostic accuracy of serologic and molecular methods for detecting visceral leishmaniasis in HIV infected patients: meta-analysis. PLoS neglected tropical diseases. 2012;6(5):e1665.
- Elmahallawy EK, Martínez AS, Rodriguez-Granger J, Hoyos-Mallecot Y, Agil A, Mari JMN, et al. Diagnosis of leishmaniasis. The Journal of Infection in Developing Countries. 2014;8(08):961-72.
- Ozerdem D, Eroglu F, Genc A, Demirkazik M, Koltas IS. Comparison of microscopic examination, rK39, and PCR for visceral leishmaniasis diagnosis in Turkey. Parasitol Res. 2009;106(1):197-200.
- Koutinas AF, Polizopoulou ZS, Saridomichelakis MN, Argyriadis D, Fytianou A, Plevraki KG. Clinical considerations on canine visceral leishmaniasis in Greece: a retrospective study of 158 cases (1989-1996). J Am Anim Hosp Assoc. 1999;35(5):376-83.
- Adel A, Berkvens D, Abatih E, Soukehal A, Bianchini J, Saegerman C. Evaluation of immunofluorescence antibody test used for the diagnosis of canine leishmaniasis in the Mediterranean Basin: a systematic review and meta-analysis. PloS one. 2016;11(8):e0161051.
- Romero HD, de Almeida Silva L, Silva-Vergara ML, Rodrigues V, Costa RT, Guimarães SF, et al. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. The American journal of tropical medicine and hygiene. 2009;81(1):27-33.
- Mettler M, Grimm F, Capelli G, Camp H, Deplazes P. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J Clin Microbiol. 2005;43(11):5515-9.
- Silva LA, Romero HD, Nascentes GAN, Costa RT, Rodrigues V, Prata A. Antileishmania immunological tests for asymptomatic subjects living in a visceral leishmaniasis-endemic area in Brazil. The American journal of tropical medicine and hygiene. 2011;84(2):261.
- Castelli G, Galante A, Verde VL, Migliazzo A, Reale S, Lupo T, et al. Evaluation of two modified culture media for Leishmania infantum cultivation versus different culture media. The Journal of parasitology. 2014;100(2):228-30.
- Lopez-Velez R, Laguna F, Alvar J, Pérez-Molina J, Molina R, Martinez P, et al. Parasitic culture of buffy coat for diagnosis of visceral leishmaniasis in human immunodeficiency virus-infected patients. J Clin Microbiol. 1995;33(4):937-9.
- Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47(1):349-58.
- Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol. 2006;44(4):1435-9.
- Galluzzi L, Ceccarelli M, Diotallevi A, Menotta M, Magnani M. Real-time PCR applications for diagnosis of leishmaniasis. Parasites & vectors. 2018;11(1):1-13.
- Merdekios B, Pareyn M, Tadesse D, Eligo N, Kassa M, Jacobs BK, et al. Evaluation of conventional and four real-time PCR methods for the detection of Leishmania on field-collected samples in Ethiopia. PLoS neglected tropical diseases. 2021;15(1):e0008903.
- Diotallevi A, Buffi G, Ceccarelli M, Neitzke-Abreu HC, Gnutzmann LV, da Costa Lima Jr MS, et al. Real-time PCR to differentiate among Leishmania (Viannia) subgenus, Leishmania (Leishmania) infantum and Leishmania (Leishmania) amazonensis: Application on Brazilian clinical samples. Acta Trop. 2020;201:105178.
- Farshchi F, Saadati A, Hasanzadeh M. Optimized DNA-based biosensor for monitoring Leishmania infantum in human plasma samples using biomacromolecular interaction: a novel platform for infectious disease diagnosis. Analytical Methods. 2020;12(39):4759-68.
- Zhang Z, Ye X, Liu Q, Hu C, Yun J, Liu R, et al. Colorimetric Nucleic Acid Detection Based on Gold Nanoparticles with Branched DNA. Nano. 2020;15(08):2050110.
- Andreadou M, Liandris E, Gazouli M, Taka S, Antoniou M, Theodoropoulos G, et al. A novel non-amplification assay for the detection of Leishmania spp. in clinical samples using gold nanoparticles. J Microbiol Methods. 2014;96:56-61.
- Jung YL, Jung C, Parab H, Li T, Park HG. Direct colorimetric diagnosis of pathogen infections by utilizing thiol-labeled PCR primers and unmodified gold nanoparticles. Biosensors Bioelectron. 2010;25(8):1941-6.
- Veigas B, Pedrosa P, Carlos FF, Mancio-Silva L, Grosso AR, Fortunato E, et al. One nanoprobe, two pathogens: gold nanoprobes multiplexing for point-of-care. J Nanobiotechnol. 2015;13(1):1-7.