NanoMicrobiol NanoBiotechnol, 2022 (1), 202211
DOI: 10.52547/nanomicrob.2022.1.1
Short Review
Iron oxide nanoparticles as a promising nanomaterials for low cost MK 7 production
Aydin Berenjian 1, 2*
1 School of Engineering, University of Waikato, Hamilton 3240, New Zealand
2 Department of Agricultural and Biological Engineering, Pennsylvania State University, University Park, PA 16802, USA
* Correspondence: aydinb@waikato.ac.nz
Abstract
Menaquinone-7 (MK-7) is produced industrially by a fermentation process of Bacillus subtilis natto at low concentrations. To date, there have been several attempts to improve MK-7 yield via optimizing the fermentation media, genetic mutation of B. subtilis natto and reducing downstream processing steps. However, it yet appears worthwhile to develop novel strategies to address the current MK-7 production challenges by developing a cost-effective fermentation process to enhance MK-7 production and reducing the fermentation process steps. In this regard, application of iron oxide nanoparticles with biocompatible coatings could provide a feasible approach for industrial production of MK‑7. This process reduces the number of downstream processing steps while enhancing the MK-7 biosynthesis which combined lead to sustainable MK-7 fermentation bioprocesses. The current short review is dealing with the potential application of iron oxide nanoparticles in the process of MK-7 production.
Keywords: Bacillus subtilis; Magnetite nanoparticles; Magnetic nanoparticles; Menaquinone-7; Natto
1. Introduction
Vitamin K exists in two forms, vitamin K1 or phylloquinone and vitamin K2 or menaquinones. Phylloquinone is present in the green leafy parts of plants, while menaquinones are produced by bacteria. All forms of vitamin K have the common 2-methyl-1, 4-naphthoquinone nucleus but they different in the length and degree of saturation of the side chain at the 3-position (1-3). Menaquinones have a variable side chain length of 4-14 repeating isoprene units referred to as MK-n where n denotes the number of isoprene units (3).
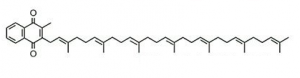
Among vitamin K series, menaquinone-7 (MK-7) is the most nutritionally recognised due to its potential in reducing the incidence of osteoporosis and preventing cardiovascular diseases besides its positive effects on blood coagulation (1, 4). Menaquinone-7 (MK-7) molecular structure is shown in the Fig. 1.
Fig. 1 Molecular structure of menaquinone-7
Several studies have been attempted to evaluate the effect of MK-7 on osteoporosis. It has been suggested that the intake of dietary MK-7 plays an important role in bone formation of individuals (5). Additionally, the review of the available epidemiological literature shows that intake of menaquinone can more effectively prevent against coronary heart disease.
Menaquinones are lipid soluble molecules that play an important role in electron transport and oxidative phosphorylation. In prokaryotes, the electron transport chain utilises MK, and thus its biosynthesis is essential for survival of B. subtilis. The biosynthesis of MK was mainly studied in Escherichia coli. Chorismate, derived from phosphoenolpyruvate (PEP) and D-erythrose-4-phosphate via the shikimate pathway, is initially converted into iso-chorismate and finally to MK through o-succinylbenzoate and 1,4-dihydroxy-2-naphthoate. These reactions are catalysed by proteins MenA to MenG.
MK biosynthetic pathway, where 1,4-naphthoquinone was converted to MK by prenylation (catalysed by MenA) followed by methylation (catalysed by MenG). Thus, the intermediate naphthoquinone to be prenylated and methylated in the new pathway was most likely 1,4-naphthoquinone 6-carboxylic acid. This compound was prepared by oxidation of naphthalene-2-carboxylic acid. This synthesised acid enabled the production of MK and recovered its growth, suggesting that 1,4-naphthoquinone-6-carboxylic acid is the biosynthetic intermediate of the new MK pathway (6, 7).
A strain of Bacillus subtilis that can be separated from the fermented food natto and so called B. subtilis natto is one of the best producers of MK-7. In industrial settings B. subtilis natto is used to produce MK-7 and efforts to make production process more efficient are undergoing. Cell immobilization is considered as a good choice for process intensification and in recent years a novel approach was introduced in this regard. This novel approach is known as magnetic immobilization and employed iron nanoparticles to immobilize the cells.
2. Iron nanoparticles
Nanoparticles are generally considered an invention of modern science. Currently, there is an intense scientific interest in nanoparticles. Nanoparticles are defined as particulate dispersions or solid particles with a size in the range of 10-100 nm or particles that have structural components smaller than 100 nm.
A wide variety of methods have been used to produce nanoparticles based on traditional and modern chemical or mechanical procedures. In general, for the solution-based synthesis of iron nanoparticles, several techniques, such as chemical precipitation, sol-gel method, forced hydrolysis, hydrothermal synthesis, electrochemical preparation, surfactant mediated or template synthesis, and emulsion/microemulsion methods, have been reported to date (8).
In general, structural and chemical stabilisation of nanoparticles are the most important requirements for a successful synthetic approach. The existence of such features ensures high surface-to-volume ratio, sufficient resistance against phase changes (e.g. oxidation) and appearance of nanoscale effects. For this reason, high-quality nanoparticle preparation methods are usually based on the use of surfactants or inorganic coatings to ensure good isolation and a series of size separation and classification procedures to minimise polydispersity (9).
A variety of materials such as proteins, polysaccharides and synthetic polymers are used for the preparation of nanoparticles. The choice of matrix material for a specific application depends on many factors including the required size of nanoparticles, surface characteristics, and the degree of biocompatibility. Among the nanoparticles, superparamagnetic nanoparticles which can be manipulated by using magnetic field gradients have shown great promise in MK-7 fermentation field. Superparamagnetic nanoparticles have unique properties such as the nearly instantaneous charge of magnetization in the applied magnetic field, which makes them responsive to an external magnetic field (10).
There are two main forms of nanoparticles that have superparamagnetic properties known as magnetite (Fe3O4) and its oxidized form maghemite (ɤ-Fe2O3). Since the superparamagnetic behaviour of iron oxide nanoparticles is strongly dependent to their dimension (11), control of uniform size distribution is very important in the synthesis. Ionic strength, pH, temperature, nature of the salts and the Fe(II)/Fe(III) concentration ratio play a key role in the size and shape of the fabricated nanoparticles (11).
In fermentation of MK-7, the downstream processes are generally involved with a large number of separation steps and therefore, at the industrial scale, the success of a bioprocess is limited by size and capital cost. Usually, downstream processing is responsible for about 60-80% of the cost of biomolecules, and therefore, is a critical factor which determines the yield and the cost of production (12). For a sustainable MK-7 bioprocess, it is therefore important, to intensify a process either through equipment intensification or process intensification. Among the many paths for process intensification lies the use of new separation strategies and the integration of reaction and separation (13).
In industrial fermentation of MK-7 using freely dispersed B. subtilis natto, bacterial cells need to be separated from the products after fermentation has taken place. Typically, the first step of MK-7 downstream processes is the cell separation step, which is carried out either by filtration or centrifugation (12) and these steps usually result in loss of viability of cells. In contrast, using the magnetic properties of IONs, the immobilized microbial cells found to be easily separated from the fermentation media without the loss of viability of the cells and the cells can then be re-used in the fermentation system.
IONs have shown a promising ability to immobilize B. subtilis natto cells without showing any negative effect on MK‑7 production and cell growth. This technology has also facilitated the in situ recovery of cells from the fermentation media with more than 95 % capture efficiency thereby reducing the number of bioprocess steps in MK-7 fermentation (14). Immobilization of the cells is, therefore, could be used to improve the productivity of MK-7 fermenting bioreactors (15). Use of IONs with appropriate coating in industrial fermentation, therefore, would offer combined advantages of submerged fermentation and re‑usability of immobilized bacterial cells as well as enhanced MK-7 production by enhancing membrane permeability and facilitating mass transfer and consequently the MK-7 production cost.
Conclusion
Considering the great demand for MK-7 and low MK-7 yield in bacterial fermentation, magnetic immobilization of B. subtilis natto cells can be used as a promising technique for biosynthesis of MK‑7. IONs could be used in the fermentation medium for further enhancing the MK-7 concentration while reducing the number of bioprocess steps in MK-7 fermentation leading to sustainable MK-7 fermentation system.
Acknowledgment
This work was supported by the University of Waikato.
References
-
- Southee R, Haroon S, Ebrahiminezad A, Ghasemi Y, Berenjian A. Novel functional fermented dairy product rich in menaquinone-7. Biocatal Agric Biotechnol. 2016;7:31-5.
- Fu X, Harshman SG, Shen X, Haytowitz DB, Karl JP, Wolfe BE, et al. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr. 2017;1(6):e000638. DOI: 10.3945/cdn.117.000638
- Berenjian A, Mahanama R, Kavanagh J, Dehghani F. Vitamin K series: Current status and future prospects. Crit Rev Biotechnol. 2015;35(2):199-208.
- Berenjian A, Mahanama R, Talbot A, Regtop H, Kavanagh J, Dehghani F. Advances in menaquinone-7 production by B. subtilis Natto: Fed-batch glycerol addition. Am J Biochem Biotechnol. 2012;8(2):105-10.
- Tsukamoto Y, Ichise H, Kakuda H, Yamaguchi M. Intake of fermented soybean (natto) increases circulating vitamin K 2 (menaquinone-7) and γ-carboxylated osteocalcin concentration in normal individuals. J Bone Miner Metab. 2000;18(4):216-22.
- Seto H, Jinnai Y, Hiratsuka T, Fukawa M, Furihata K, Itoh N, et al. Studies on a new biosynthetic pathway for menaquinone. J Am Chem Soci. 2008;130(17):5614-5.
- Hiratsuka T, Furihata K, Ishikawa J, Yamashita H, Itoh N, Seto H, et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science. 2008;321(5896):1670-3.
- Mohapatra M, Anand S. Synthesis and applications of nano-structured iron oxides/hydroxides – A review. International Journal of Engineering, Science and Technology. 2010;2(8):127-46.
- Simeonidis K, Mourdikoudis S, Kaprara E, Mitrakas M, Polavarapu L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. E Int J Eng Sci Technol. 2016;2(1):43-70.
- Ihor T, Sergiy M, Mikhail M, Yuri R. Colloidal Systems on the Nanometer Length Scale. Handbook of Surface and Colloid Chemistry, Third Edition: CRC Press; 2008. p. 131-54.
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108(6):2064-110.
- Sirkar KK, Luo RG, Xu Y, Dai X-P, inventors; Google Patents, assignee. Method and apparatus for isolation and purification of biomolecules2006 Jan. 17, 2006.
- Vaghari H, Eskandari M, Sobhani V, Berenjian A, Song Y, Jafarizadeh-Malmiri H. Process intensification for production and recovery of biological products. Am J Biochem Biotechnol. 2015;11(1):37.
- Ebrahiminezhad A, Varma V, Yang S, Berenjian A. Magnetic immobilization of B. subtilis natto cells for menaquinone-7 fermentation. Appl Microbiol Biotechnol. 2016;100(1):173-80.
- Akay G, Erhan E, Keskinler B. Bioprocess intensification in flow‐through monolithic microbioreactors with immobilized bacteria. Biotechnol Bioeng. 2005;90(2):180-90.