NanoMicrobiol NanoBiotechnol, 2023 (1), 202313
DOI:
Original Article
Functionalization of chitosan nanofibers with zinc oxide nanoparticles
Zahra Asvar 1, Esmaeel Mirzaee 1, and Alireaza Ebrahiminezhad 2*
1 Department of Medical Nanotechnology, School of Advanced Medical Sciences and Technologies, Shiraz university of Medical Sciences, Shiraz, Iran
2 Biotechnology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
* Correspondence: a_ebrahimi@sums.ac.ir
Abstract
In recent years, nanofibers were emerged as one of the most popular wound dressing. Specially in the case of infected wounds or burn ulcers that are infected with multidrug resistant bacteria antimicrobial nanofibers can be a promising choice. Functionalization with antimicrobial nanoparticles is an efficient approach for the fabrication of antimicrobial nanofibers. Zinc oxide nanoparticles are well-known as biocompatible antimicrobial nanoparticles that can be employed in the antimicrobial wound dressing. But, functionalization of nanofibers with zinc oxide nanoparticles is not such a simple. In this experiment in situ reduction of zinc ion was performed to fabricate zinc oxide functionalized chitosan nanofibers. Feasibility of the in situ reduction was examined by using two different reducing agents, hydrazine and sodium hydroxide. It was found that hydrazine is not able to reduce zinc nanoparticles inside chitosan nanofibers. But, in situ formation of zin oxide nanoparticles was stimulated by using a solution of sodium hydroxide. Based on TEM micrographs, diameter of the prepared nanofibers was about 50-200 nm. Treatment with sodium hydroxide to some extent disturb the structure of nanofibers. To reach the best possible result it seems that some optimization study in regard to concentration of sodium hydroxide solution and other parameters should be performed.
Key words: Antimicrobial nanoparticles; Antimicrobial nanofibers; Electrospinning; Wound dressing; Metal nanoparticles
1 Introduction
Nanofibers are ideal choices for wound dressing because of their resemblance to the skin’s extracellular 3D matrix. Nanofibers porous structure can facilitate the exchange of gases and nutrients while prevent wound drying (1, 2). At the same time, the complex structure of nanofibers network and the size of its cavities do not allow microorganisms to reach the wound surface. As a result, the likelihood of developing a wide range of bacterial, fungal and viral infections will be significantly reduced (3, 4). Also, creating a high reactivity level of these graphs is conducive to loading and delivery of drugs, biomolecules and nanoparticles, which makes nanofibers a suitable option for use in a variety of wound coatings (2, 5).
Several methods have been developed for the production of nanofibers, the main of which are drawing, self-assembly, phase separation, synthesis template, and electrospinning (6). Electrospinning is a method of producing fiber at the nanoscale using electrostatic force, which was known from the early 1930s, but was not considered until the 1990s, when it was proved that a large number of organic polymers were capable of electrospinning with nanometer sizes (7). Nanofibers are now commonly produced through electrospinning technique, which is a simple, cost-effective, repeatable and high-variety method in selecting raw materials and controlling the final product with excellent imitation of extracellular matrix (8). In fact, electrospun nanofiber matrixes accelerate wound healing due to the absorption of secretions from the wound, the possibility of exchanging respiratory gases and water vapor, providing a moist environment on the wound, and preventing microbial infections. This has led to special attention in recent years for use as wound coverings and dressings. The possibility of producing these bandages on an industrial scale can be another reason to pay special attention to this method in the production of nanofibers (9).
In recent years, production of antibacterial wounds dressing using natural and synthetic nanofibers along with nanoparticles such as gold, silver, zinc oxide, copper oxide, titanium oxide, and iron oxide has attracted much attention for treatment of infectious wounds. To prevent bacterial infections, a suitable antibacterial substance with minimal destructive effects and stable antibacterial properties for a wide range of bacteria would be an ideal option. Zin oxide nanoparticles can be one of these ideal options. Safety and compatibility of zinc oxide with human skin makes it a suitable additive for textiles and other products that are in contact with the human body. Incorporation of these nanoparticles in the nanofibers can also improve physical properties of the fibers. For instance, it has been show that an optimum concentration (3 wt%) of zinc oxide nanoparticles would increase the toughness of the scaffold and improved tensile strength and Young’s modulus. Also, relatively low concentrations of nanoparticles inhibited the growth of Staphylococcus aureus and Escherichia coli (10). In another study, Augustine and his colleagues investigated the effect of zinc oxide nanoparticles on diameter, morphology, antibacterial activity and cell proliferation of pelicaprolectone electrolysis. This study showed an increase in mechanical stability, antibacterial properties, fibroblast proliferation, and improvement of metabolic activity of the cells. This was the first report on the ability of a biomaterial containing zinc oxide nanoparticles to increase cell proliferation (11). Another team of researchers in 2018 embedded zinc oxide and zinc acetate nanoparticles in polycaprolactone nanofibers to create antibacterial nanocomposite wound dressings against E. coli and S. aureus bacteria. The effect of UV radiation on the antibacterial activity of nanofibers was also studied in this study. Results showed that nanofibers were able to prevented the growth of planktonic cells and bacterial biofilms. This capability was found to be due to release of zinc ions (mainly from zinc acetate nanoparticles) and photocatalytic oxidative processes. Antibacterial properties of the samples were also significantly increased by UV irradiation (12). In 2021, Bagheri and her colleagues synthesized nanofibers that was made of chitosan and PEO polymers with silver and zinc nanoparticles for wound dressing application. Resulted fibers was claimed to provide antioxidant effect and also high antibacterial activity against E. coli, S. aureus and Pseudomonas aeruginosa. This nanocomposite had no toxicity on fibroblast cells based on cellular toxicity studies (13). Beside all these performed study, functionalization of electrospun nanofibers with zinc oxide nanoparticles is not still a simple procedure. In situ reduction of nanoparticles is one of the promising and mostly employed approaches toward fabrication of metal nanoparticles functionalized fibers. So, in the current experiment we examined two different reducing agents (hydrazine and sodium hydroxide) for the in situ reduction of zinc oxide nanoparticles in chitosan electrospun nanofibers. Resulted data can lead us toward simple process for the fabrication of nanofibers which are functionalized with valuable zinc oxide nanoparticles.
2 Experimental
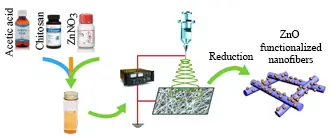
In the current experiment we developed a simple technique for the fabrication of silver enriched chitosan nanofibers. The technique was based on post electrospinning silver functionalization and silver ion reduction to silver nanoparticles. In brief, chitosan (0.14 g), PEO (0.035), and zinc citrate (0.1 mM) were dissolved in acetic acid (7 mL). The solution was subjected to electrospinning by using an electrospinning set up (NanoAzma, Full Option Lab ES II, Tehran, Iran). Electrospinning parameters were set as injection rate 1 mL/h, voltage 18 Kv, rotation 200 rpm, distance 14 cm. To make the fibers water resistance, resulting fibers were incubated in the PSB (pH 7.4) vapor at 60°C. To prevent water loos, the fibers were maintained above PBS solution in a sealed container and container was incubated in an oven. After two-hour incubation time the fibers were submerged in reducing solution. Two reducing solutions were examined NaOH (1 M) and hydrazine (0.16%) in ethanol. The fibers were immersed in the reduction solution for about five minutes. Fibers were rinsed with deionized water and were died at ambient atmosphere. Resulting fibers were evaluated by using a transmission electron microscopy (TEM, Zeiss-EM10C-100 KV, Germany) without any sample preparation. Diameters of the nanofibers were measured on the resulted TEM micrographs via an image analyzing software (ImageJ 1.53t) that was developed by the National Institutes of Health (NIH).
3 Results and discussion
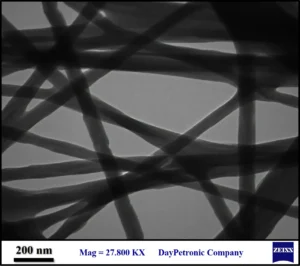
Electrospinning of chitosan solution resulted in a flexible thin layer of fibers. Micrograph of the chitosan nanofibers was provided as Fig. 1. As can be seen the resulted fibers were ~50-200 nanometer in diameter with any grain or rough surface. TEM micrograph of the zinc enriched chitosan fibers which were subjected to reduction with hydrazine was provided as Fig. 2. The fibers were not so different with the chitosan fibers that were prepared without zinc. The fibers were also about 50 nm to 200 nm in diameter with no grain and smooth surface. This feature indicated that nanoparticles were not formed during reduction by hydrazine. Zinc enriched fibers were also reduced in sodium hydroxide and corresponding TEM image was depicted as Fig. 3. These fibers exhibit a grained appearance and also surface of the fibers was rough to some extent. Zinc oxide nanoparticles can be seen as spherical dark objects that are imbedded within the fibers. Also, some amorphous small particles were formed outside the fibers.
Fig. 1 TEM micrograph of the chitosan nanofibers
Fig. 2 TEM micrograph of the zinc enriched chitosan nanofibers that were treated with hydrazine solution
Fig. 3 TEM micrograph of the zinc enriched chitosan nanofibers that were treated with sodium hydroxide
These findings indicated that sodium hydroxide was able to stimulate the formation of zinc oxide nanoparticles within zinc enriched fibers. To prevent any possible diffusion of zinc ions out of fibers during reduction process, the reduction solutions were prepared based on organic solvent (ethanol). But it seems that there was some leakage of zin ions that resulted in the formation of small nanoparticles out of the fibers (Fig. 1). These results are in close agreement with the previous reports that were shown the potential of alkaline solutions for the synthesis of zinc oxide nanoparticles (14). The major disadvantage of sodium hydroxide was its adverse effect on the fibers structure. It was seen that treatment with sodium hydroxide can disturb the structure of nanofibers (Fig. 4). By optimizing the concentration of sodium hydroxide it can be possible to reduce or eliminate the adverse effects of reduction process.
Fig. 4 Adverse effect of sodium hydroxide on the structure of chitosan nanofibers
Conclusion
In this experiment two procedures were examined for the functionalization of chitosan nanofibers with zinc oxide nanoparticles. It was founded that employed reducing agent has key effect on the successful functionalization. In the nanofibers matrix, hydrazine which is a well-known reducing agent was not able to reduce zinc ion to zinc oxide nanoparticles. But, sodium hydroxide was able to stimulate the formation of zinc oxide nanoparticles. Obviously, optimization studies should be done to reveal the best concentration of sodium hydroxide and reduce its adverse effect on the nanofibers structure.
References
-
- Dong Y, Zheng Y, Zhang K, Yao Y, Wang L, Li X, et al. Electrospun nanofibrous materials for wound healing. Advanced Fiber Materials. 2020;2(4):212-27.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. Journal of International Medical Research. 2009;37(5):1528-42.
- Liu X, Lin T, Fang J, Yao G, Zhao H, Dodson M, et al. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. Journal of biomedical materials research Part A. 2010;94(2):499-508.
- Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites science and technology. 2003;63(15):2223-53.
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: Current advances and future directions. Journal of Applied Polymer Science. 2019;136(27):47738.
- Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. International Journal of nanomedicine. 2006;1(1):15.
- Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Advanced materials. 2004;16(14):1151-70.
- Liu M, Duan X-P, Li Y-M, Yang D-P, Long Y-Z. Electrospun nanofibers for wound healing. Materials Science and Engineering: C. 2017;76:1413-23.
- Chen S, Liu B, Carlson MA, Gombart AF, Reilly DA, Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017;12(11):1335-52.
- Rodríguez-Tobías H, Morales G, Ledezma A, Romero J, Grande D. Novel antibacterial electrospun mats based on poly (d, l-lactide) nanofibers and zinc oxide nanoparticles. Journal of materials science. 2014;49(24):8373-85.
- Augustine R, Malik HN, Singhal DK, Mukherjee A, Malakar D, Kalarikkal N, et al. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. Journal of Polymer Research. 2014;21(3):1-17.
- Prado-Prone G, Silva-Bermudez P, Almaguer-Flores A, García-Macedo JA, García VI, Rodil SE, et al. Enhanced antibacterial nanocomposite mats by coaxial electrospinning of polycaprolactone fibers loaded with Zn-based nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14(5):1695-706.
- Bagheri M, Validi M, Gholipour A, Makvandi P, Sharifi E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioengineering & Translational Medicine. 2021:e10254.
- Koutu V, Shastri L, Malik M. Effect of NaOH concentration on optical properties of zinc oxide nanoparticles. Materials Science-Poland. 2016;34(4):819-27.