NanoMicrobiol NanoBiotechnol, 2023 (1), 202313
DOI:
Original Article
Effects of reaction parameters on the optical activity of biosynthesized silver nanoparticles
Mahboubeh Bagheri 1 and Seyedeh-Masoumeh Taghizadeh 2*
1 Department of pharmaceutical Biotechnology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz Iran
2 Biotechnology Research Center; Shiraz University of Medical Sciences, Shiraz Iran
* Correspondence: taghizm@sums.ac.ir
Abstract
Nowadays, the use of metal nanoparticles in different sciences is increasing. Silver nanoparticles are among the most widely used nanoparticles, especially in the field of medical sciences. There are many physical and chemical methods for the synthesis of silver nanoparticles, but these methods are inefficient due to the use of high temperature, high pressures, harsh pH, and the use of toxic substances. Therefore, nowadays, efforts for biological synthesis of silver nanoparticles are increasing. Reaction parameters has significant effect on the characteristic feature of resulted AgNPs. One of these important characteristic feature is optical activity that made AgNPs applicable in diverse optical techniques. So, in the current experiment the effect of reaction parameters such as silver nitrate concentration, amount of biological reducing compounds, and reaction temperature on the optical activity of resulted AgNPs was investigated. It was found that 5 mM silver nitrate, reaction temperature 50°C, and culture supernatant ratio of 1:2 can provide the best result. AgNPs that were synthesized at such condition was 5-14 nm in size with 7 nm average diameter and provide the most possible optical activity.
Key words: Biosynthesis; Green synthesis; Metal nanoparticles; Optical activity; Surface plasmon resonance
1 Introduction
Silver nanoparticles (AgNPs) were introduced as powerful antimicrobial nanostructures that gained vast applications in science, technology, and commercial products. Due to expanded applications of this nanoparticles, the technology for the fabrication of AgNPs has been developed much more that other nanostructures. Top down and bottom up techniques were developed in this regard. But, due to high energy demand and low quality of resulted nanoparticles, the top down approach is not so in use. Wet chemistry and fabrication of nanoparticles via sedimentation reactions are more in spot light. Now AgNPs can be synthesized via chemical reactions by using chemical reducers such as sodium borohydride, hydrazine, sodium citrate, and also biochemical reducers such as reducing sugars (1, 2).
Beside chemical synthesis of AgNPs, the green approaches were also developed in this regard. Fabrication of AgNPs in aqueous media by using biological and safe compounds were developed drastically. Now numerus plants and microbial cells have been used for the biosynthesis of AgNPs (3-5). Chlorella vulgaris microalgae is one of the microbial cells that was employed in different approaches for the biosynthesis of AgNPs (6, 7).
vulgaris is a green microalgae residing in freshwater that is classified by the Food and Drug Administration in the category of safe and was approved for pharmaceutical and medicinal applications. This microalga is grown in industrial scales and its processed biomass is available as a dietary supplement. Culture supernatant is the result of this microalgae large scale cultivation and has any specific application and considered as waste material. Previous studies have shown that secreting materials from C. vulgaris cells that accumulated in the supernatant are able to drive the reactions of nanoparticle synthesis. It has been shown that nanoparticles synthesized in the supernatant obtained from the culture of this microalgae had a very suitable particle size distribution and a completely uniform shape (7, 8). Previous studies have shown that secreted carbohydrates of chlorella vulgaris are the main compound found in supernatant culture of this microalgae which is able to control the synthesis reaction of nanoparticles (7, 9).
Beside the secretory compound, it has been shown that cell extract of C. vulgaris is also able to perform reduction of silver ions to form silver nanoparticles. It was found that cell proteins are the effective biomaterial in the cell extract that can drive the reaction (6). However, in contrast to cell extract that is a valuable and applicable compound in pharmaceutical application, culture supernatant is a waste of microalgae producing plants and is considered as useless by product. Therefore, culture supernatural of C. vulgaris microalgae can be a suitable option for the economic synthesis of nanoparticles with medical applications. Because on the one hand, the use of this microalga has been permitted by the Food and Drug Administration for pharmaceutical products, and on the other hand, the use of culture supernatant will not be costly.
It is obvious that reaction parameters such as concentration of silver precursor, reaction temperature, amount of bioactive compounds, and reaction time have an immense impact on the biosynthesis of AgNPs (10). There is no data about the effects of reaction parameters on the biosynthesis of AgNPs by using secretory compound from C. vulgaris. So, in the current experiment the effects of reaction parameters on the optical activity of resulted AgNPs was investigated.
2 Materials and methods
2.1 Materials
Silver nitrate was from Merck (Darmstadt, Germany). BG-11 media was prepared based on previous reports (7). C. vulgaris was brought from Department of Pharmaceutical Biotechnology, Pharmacy School, Shiraz University of Medical Sciences.
2.2 Culture of C. vulgaris
Based on previous investigations the C. vulgaris cells (E7 cells/mL) were culture in BG-11 media and incubated at 28°C under continues illumination using white light. The growth of microalgal cells was evaluated throughout 20 days by direct cell count. After 20 days, the culture was centrifuged (13000 rpm, 10 min) and resulted supernatant was employed as culture supernatant for the biosynthesis of AgNPs.
2.3 Biosynthesis of AgNPs
In order to investigate the effect of temperature, 400 μl of microalgae culture supernatant was added to 1600 μl of silver nitrate solution. The final concentration of silver nitrate in reaction mixture was 1 mM and the ratio of supernatant to silver nitrate solution was 1:4. The prepared solutions were stored at 40, 30, 25 and 50 °C for 24 hours without any shaking or agitation.
To investigate the effect of silver nitrate concentration, reaction solutions were prepared with microalgae culture supernatant ratio to silver nitrate solution of 1:4 in which the final concentration of silver nitrate was 1, 5 and 10 mM. The mixture was stored at 30°C for 24 hours without any shaking or agitation.
To investigate the effect of ratio of culture supernatant to silver nitrate solution, reaction solutions with ratios of 1:1, 1:2, 1:4 and 1:10 with the final concentration of silver nitrate of 1 mM were prepared and stored at 30°C for 24 hours.
For each experiment, blank reaction was considered without any silver nitrate. After incubation time, optical activities of all samples were measured in the range of 300 to 700 nm.
3 Results
3.1 Culture of C. vulgaris cells
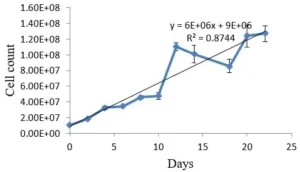
Growth of the C. vulgaris cells was evaluated by direct cell count with a Neubauer chamber for 20 days. The resulted growth curve was shown in the Fig. 1.
Fig. 1 Growth curve of C. vulgaris cells over 20 days cultivation in BG-11 medium
3.2 Biosynthesis of AgNPs
3.2.1 Effect of silver nitrate
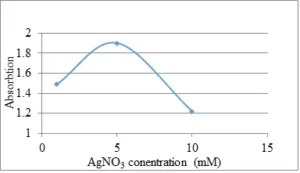
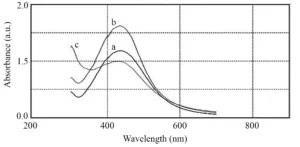
In order to evaluate the effects of silver nitrate concentration in the biosynthesis of AgNPs, increasing concentrations of silver nitrate (1, 5 and 10 mM) were investigated. As can be seen in Fig. 2, the highest optical absorption of the nanoparticles was achieved at 5 mM silver nitrate. With increasing concentration up to 5 mM, the optical absorption intensity increases, indicating an increase in the reduction of silver ions and biosynthesis of AgNPs. However, with increasing the concentration of silver nitrate to 10 mM, a decrease in the height of the adsorption peak was observed. At concentrations of 1 and 5 mM the absorption peak was at 434 nm and at the concentration of 10 mM was recorded at 428 nm (Fig. 3). Accordingly, it can be said that the synthesized nanoparticles at a concentration of 10 mM of silver nitrate have a smaller particle size than the synthesized nanoparticles at concentrations of 1 and 5 mM. Therefore, because 5 mM silver nitrate provided considerably more AgNPs, it can be concluded that the optimum concentration of silver nitrate for biosynthesis of AgNPs is 5 mM.
Fig. 2 Optical activity of resulted AgNPs which were synthesized in different concentration of silver nitrate
Fig. 3 UV-vis spectra of the AgNPs which were synthesized at 1 mM (a), 5 mM (b), and 10 mM (c) silver nitrate
3.2.2 Effect of culture supernatant
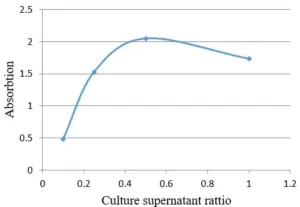
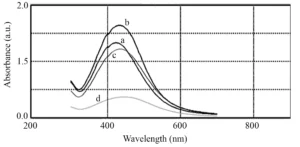
Regarding the effect of microalgae culture supernatant ratio (as reducing solution) to silver nitrate solution, it is observed that by decreasing the amount of microalgae culture supernatant, the optical absorption curve of the microalgae shifts red, indicating an increase in the particle size of the synthesized nanoparticles. As can be seen in Fig. 4 and Fig. 5, the synthesis rate of nanoparticles in the ratio of 1:2 from microalgae culture to silver nitrate solution is higher than other ratios and increases optical absorption. Since the width of the peaks is not very different, it can be concluded that the particle size distribution of synthesized nanoparticles in all the tested ratios is almost the same.
3.2.3 Effect of culture supernatant
Regarding the effect of microalgae culture supernatant ratio (as reducing solution) to silver nitrate solution, it is observed that by decreasing the amount of microalgae culture supernatant, the optical absorption curve of the microalgae shifts red, indicating an increase in the particle size of the synthesized nanoparticles. As can be seen in Fig. 4 and Fig. 5, the synthesis rate of nanoparticles in the ratio of 1:2 from microalgae culture to silver nitrate solution is higher than other ratios and increases optical absorption. Since the width of the peaks is not very different, it can be concluded that the particle size distribution of synthesized nanoparticles in all the tested ratios is almost the same.
Fig. 4 Effect of culture supernatant ratio to silver nitrate solution on optical activity of AgNPs
Fig. 5 UV-vis spectra of the biosynthesized AgNPs at different culture supernatant to silver nitrate ratio, 1:1 (a), 1:2 (b), 1:4 (c), and 1:10 (d)
3.2.4 Effect of reaction temperature
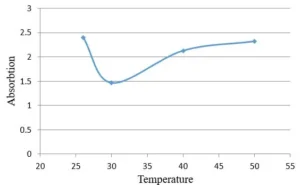
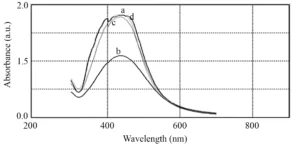
To determine the effect of reaction temperature on the formation of silver nanoparticles, reduction reaction was carried out at a temperature range of 25-50°C (Fig. 6). The optical absorption spectrum of nanoparticles obtained at different temperatures does not show a clear difference in width and peak position (Fig. 7). Therefore, it can be concluded that the reaction temperature has no effect on the particle size distribution of synthesized nanoparticles and only affects the rate of AgNPs biosynthesis. The synthesis rate of nanoparticles and consequently peak height at 25°C and 50°C is higher than other temperatures, but the peak width at all these temperatures is almost the same and the peak location at all temperatures is about 434 nm.
Fig. 6 Optical activity of AgNPs that were synthesized at different temperatures
Fig. 7 UV-vis absorption of silver nanoparticles synthesized at 25°C (a), 30°C (b), 40°C (c), and 50°C (d)
3.3 TEM imaging
AgNPs were synthesized at optimal reaction condition, ratio of culture supernatant to silver nitrate 1:2, silver nitrate final concentration was 5 mM, and reaction temperature was 50 °C for 24 hours. Optical activity of the AgNPs that were synthesized in the optimal condition was shown in Fig. 8. TEM image of the nanoparticles was also shown in Fig. 9. Based on the analysis which were conducted on the TEM image, most of the synthesized nanoparticles are spherical and the size of nanoparticles was 5 nm to 14 nm. The average size of nanoparticles was 7 nm.
Fig. 8 UV-vis spectra of the AgNPs that were synthesized in the optimal condition, spectrum was recorded after 10-time dilution
Fig. 9 TEM image of the AgNPs which were fabricated at optimal reaction condition
4 Discussion
AgNPs are one of the most common and widely used nanoparticles. The formation of silver nanoparticles by different bacteria and fungi as well as extracts of various plants is well known. However, the ability of microalgae to synthesize silver nanoparticles is not well known. Kalimuthu and his colleagues have succeeded in synthesizing silver nanoparticles with appropriate particle size distribution and particle size of about 50 nm by adding silver nitrate solution to Bacillus licheniformis cell mass. They reported that the cell mass obtained in the static phase had the highest production rate of nanoparticles (45). Faghrizonoon and his colleagues reported that the addition of 1 mM silver nitrate solution to the solution of Streptomyces sp. ERI-3 after 48 hours of incubation in the dark resulted in the formation of spherical AgNPs with a size of 10 to 100 nm (46). Bhainsa and his colleagues reported extracellular synthesis of AgNPs (5 to 25 nm) by Aspergillus fumigates (47). Fusarium solani fungus has been successfully used for extracellular synthesis of silver spherical nanoparticles with a wide particle size distribution ranging from 5 to 35 nm (48). Mahdieh and his colleagues reported the synthesis of medium-sized AgNPs of 11.6 nm using spirulina platensis cyanobacteria (49). In the present study, we succeeded in synthesizing AgNPs using culture supernatant of a unicellular alga. It was observed that after adding silver ion with the final concentration of 5 mM to the solution of microalgae medium, the color of the medium changed to red-brown after a few minutes. The appearance of brown color indicates the formation of AgNPs due to the revival of silver to metallic silver ions. The reduction of silver ions is probably carried out by regenerative agents secreted into the culture medium by microalgae cells (50). There are several possible mechanisms for the synthesis of AgNPs by microalgae. The most likely mechanism is that cell reductase secreted into the culture medium by microalgae cells is involved in the reduction of metal ions to nanoparticles (49). In the case of cyanobacteria, the NADH cofactor secreted in the medium plays an important role in this process, and NADH-dependent reductase can be responsible for the reduction of metal ions. It has been reported that some NADH-dependent reductases are involved in the formation of AgNPs by using Fusarium oxysporum fungus (48). It has been observed that regenerative agents secreted in environments such as hydroquinones are able to reduce metal ions to nanoparticles (50, 51). Therefore, electron transporters or other regenerative agents secreted by microalgae may be able to reduce silver ions to AgNPs. Reductase enzymes in microalgae (37) may also be involved in the synthesis of metal nanoparticles. This enzyme is an electron transporter between nitrate and metals (52). Reductase enzyme has already been purified and used for extracellular synthesis of AgNPs (45, 52). Barwal and his colleagues found that different cell proteins in Chlamydomonas reinhardtii such as histone, carbonic anhydrase, ferredoxin NADP+ reductase, superoxide dismutase, ATP synthase, sedohptoptolose-1 and -bisphosphates are associated with synthesized AgNPs but are not associated with pre-synthesized nanoparticles. Reduction in the ratio of these proteins leads to changes in the size and speed of biosynthesis of AgNPs. These observations confirm that these proteins have direct control over the biosynthesis of AgNPs in this microalgae (53). However, further studies should be conducted to determine the exact mechanism involved in the biosynthesis of AgNPs by microalgae. One of the advantages of the present work is that the synthesized nanoparticles have a small size in the range of 5 to 15 nm, so these nanoparticles do not block kidney glomerulus when used in medical applications and are likely to be easily excreted through urine in a short time (54). The absorption spectrum of the reaction solution containing silver ions showed an increase in adsorption intensity of about 420 nm and after about 2 hours a clear peak was observed. Increase in optical absorption intensity in an area of 400 to 450 nm over time can be due to increased synthesis of AgNPs due to the revival of silver ions. Stability is one of the most important aspects of nanoparticle synthesis. Instability of many products made from nanoparticles has limited the development of their applications. In order to evaluate the stability of synthesized nanoparticles, suspension of nanoparticles was stored at room temperature for six months. The optical absorption spectrum of this suspension, which is prepared at different times after its storage at room temperature, shows that over time the height of the absorption peak increases. This reflects the fact that, over time, even after 6 months (180 days), the process of reduction of residual silver ions in the reaction solution continues and new nanoparticles are being formed, leading to a significant increase in the color of the reaction solution as well as an increase in optical absorption. Over time, the optical absorption peak of nanoparticles shifts slightly toward higher wavelengths. Shifts to higher wavelengths (red shifts) occur due to increased size of nanoparticles or accumulation (aggregation) of nanoparticles. In order to investigate the aggregation probability of nanoparticles, the optical absorption spectrum of nanoparticles stored at room temperature for 25 days before and after sonication was investigated (Fig. 25-3). Sonication causes these aggregations to be discrete and the nanoparticles to become monodispersed again. The optical absorption spectrum of AgNPs after sonication shows a blue shift compared to their spectrum before sonication. These findings indicate that by keeping AgNPs at room temperature for a long time, somewhat aggregation of nanoparticles is done. However, no deposition or particulate matter was observed in the suspension of nanoparticles even after 6 months. These findings show the acceptable stability of synthesized nanoparticles of this microalgae.
Conclusion
Microalgae C. vulgaris was employed successfully for the fabrication of AgNPs. It was revealed that reaction parameters such as reaction temperature, concentration of silver precursor, and amount of biological compound as reducing agents have significant effects on the optical activity of resulted nanoparticles. These data indicate the importance of reaction optimization in biosynthesis of AgNPs.
References
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of AgNPs: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385-406.
- Khan Y, Nasar M, Numan M, Ullah I, Shinwari Z. Biomimetic Synthesis of AgNPs for Breast Cancer Therapeutics and Its Mechanism. International Journal of Nanotechnology and Nanomedicine. 2018;3(1):1-9.
- Taghizadeh S, Taghizadeh S-M, Ghasemi Y, Ebrahiminezhad A. Living Plant-Mediated Synthesis of Nanoparticles. Journal of Advanced Medical Sciences and Applied Technologies. 2018;4(1):1-6.
- Siddiqi KS, Husen A, Rao RAK. A review on biosynthesis of AgNPs and their biocidal properties. J Nanobiotechnol. 2018;16(1):14.
- Chandraker SK, Ghosh MK, Lal M, Shukla R. A review on plant-mediated synthesis of AgNPs, their characterization and applications. Nano Express. 2021;2(2):022008.
- Xie J, Lee JY, Wang DI, Ting YP. Silver nanoplates: from biological to biomimetic synthesis. ACS Nano. 2007;1(5):429-39.
- Ebrahiminezhad A, Bagheri M, Taghizadeh S-M, Berenjian A, Ghasemi Y. Biomimetic synthesis of AgNPs using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv Nat Sci. 2016;7(1):015018.
- Ghanbariasad A, Taghizadeh S-M, Show PL, Nomanbhay S, Berenjian A, Ghasemi Y, et al. Controlled synthesis of iron oxyhydroxide (FeOOH) nanoparticles using secretory compounds from Chlorella vulgaris microalgae. Bioengineered. 2019;10(1):390-6.
- Taghizadeh S-M, Lal N, Ebrahiminezhad A, Moeini F, Seifan M, Ghasemi Y, et al. Green and economic fabrication of zinc oxide (ZnO) nanorods as a broadband UV blocker and antimicrobial agent. Nanomaterials. 2020;10(3):530.
- Taghizadeh S-M, Zare-Hoseinabadi A, Berenjian A, Ghasemi Y, Ebrahiminezhad AJJoETT. Effective Parameters in the Green Synthesis of Zero-valent Iron Nanoparticles as a Fenton-like Catalyst. Journal of environmental treatment techniques. 2020;8(1):442-7.